Quality Policy
Quality Policy
Quality Control
The Quality Control system is an integral part of cGMP and ensures that the necessary and relevant tests are done and that neither the material not the products are released for use or supply, until their quality has been judged to be satisfactory.
The Quality Control Laboratory is responsible for sampling and testing of all starting materials, and packaging materials in accordance to the predetermined specifications and established procedures. In case of raw materials, 100% sampling is carried out for identification and composites samples are tested as per the specifications. The QC laboratory is also responsible for testing of in- process and finished product samples. The stability studies are carried out at Quality Control Laboratory.
The Microbiological testing includes Assays for vitamins, sub-culturing of different micro-organisms, Viable Environmental Monitoring of Non Aseptic areas and Sampling & Analysis of different grades of water. The Quality Control laboratory is fully equipped with most sophisticated and modern analytical instruments for chemical and instrumental analysis.

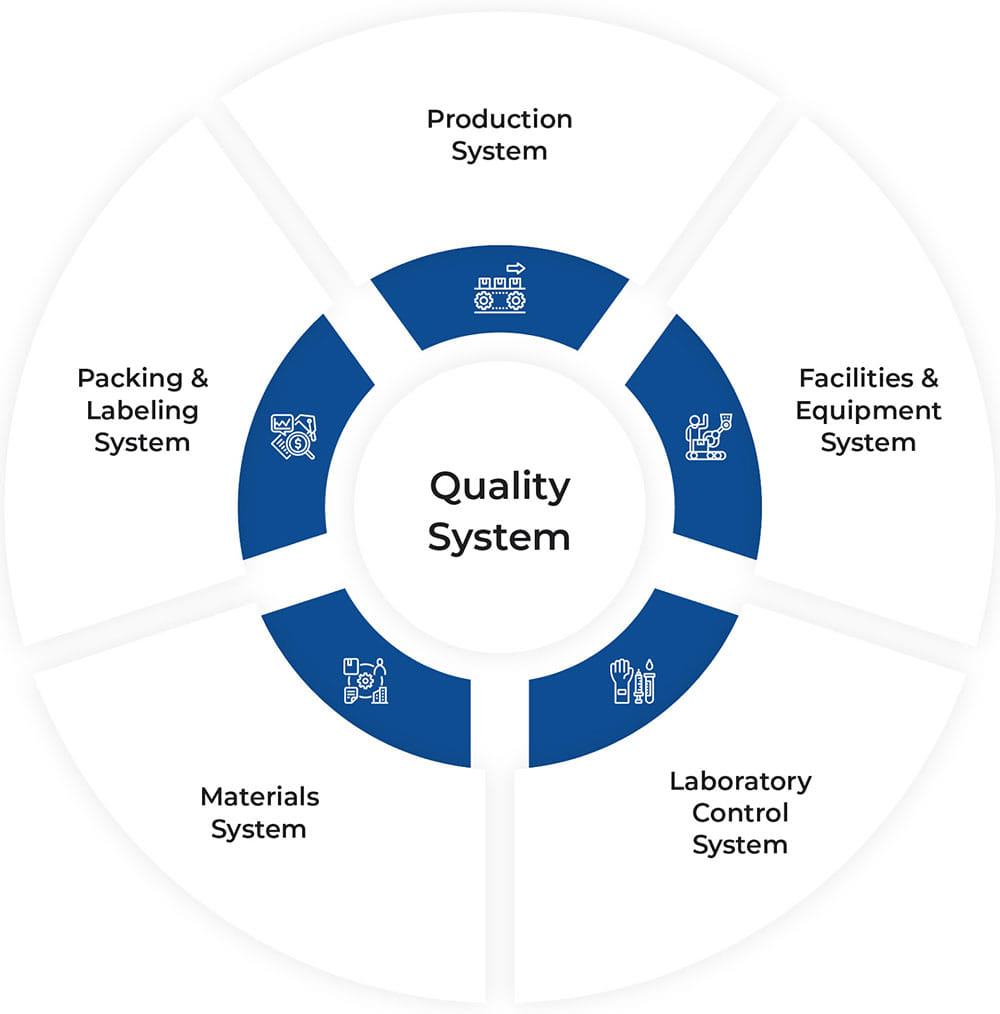
Quality Assurance
The Quality Management System is governed by the Principles and Requirements as defined in the Schedule M of Indian Drugs and Cosmetics Acts 1940, as well as cGMP. The Quality management system is established with an understanding that “Product Quality as well as performance quality” are crucial in the long term survival of the organization.
The Quality management system stresses upon key aspects viz., process capability operating system acceptance supply and quality motivation. The purpose of the Quality Policy is to ensure the compliance of Quality systems and procedures so that the Finished Drug Products manufactured at site meets all the required specifications ensuring the identity, strength, safety and purity of products.
The Quality Control Department is independent from manufacturing and authorized to take appropriate decisions on quality matters of Raw material / Packaging material Finished products or any other issues related to quality.






